|
|
MARIA™ - The next generation of allergen detection systems
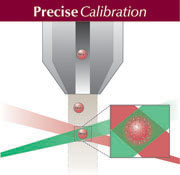 Indoor Biotechnologies has developed a Multiplex ARray for Indoor Allergens (MARIA™) that measures the eight most important indoor allergens at once in a single test. The array simultaneously measures Der p 1, Der f 1, mite Group 2, Fel d 1, Can f 1, Rat n 1, Mus m 1 and Bla g 2. MARIA™ provides improved assay performance (increased sensitivity, accuracy and precision) in a high throughput system with substantial time and cost savings. Indoor Biotechnologies has developed a Multiplex ARray for Indoor Allergens (MARIA™) that measures the eight most important indoor allergens at once in a single test. The array simultaneously measures Der p 1, Der f 1, mite Group 2, Fel d 1, Can f 1, Rat n 1, Mus m 1 and Bla g 2. MARIA™ provides improved assay performance (increased sensitivity, accuracy and precision) in a high throughput system with substantial time and cost savings.
MARIA™ technology was developed in collaboration with Radix Biosolutions and Luminex Corporation, with support from a Small Business Innovation Research (SBIR) contract from the National Institutes of Environmental Health Sciences (NIEHS). MARIA™ combines Indoor Biotechnologies proprietary allergen-specific monoclonal antibodies with the power of Luminex xMAP® technology and represents the state-of-the-art in allergen detection.
MARIA™ kits and services are available exclusively through Indoor Biotechnologies. We hope that you will try MARIA™ kits and services for allergen exposure assessment and welcome your feedback on this exciting new technology. |
| What is MARIA™ technology? |
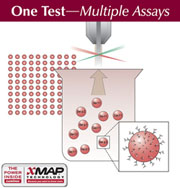 MARIA™ incorporates Indoor Biotechnologies monoclonal antibodies on the Luminex xMAP® system. The antibodies are covalently coupled to polystyrene beads containing different fluorescent dyes. Each bead set functions as a solid phase for a specific allergen, which binds to the bead and is detected using biotinylated antibody and streptavidin-phycoerythrin. The fluorescent intensity is measured in a Luminex Instrument and is directly proportional to allergen concentration. MARIA™ incorporates Indoor Biotechnologies monoclonal antibodies on the Luminex xMAP® system. The antibodies are covalently coupled to polystyrene beads containing different fluorescent dyes. Each bead set functions as a solid phase for a specific allergen, which binds to the bead and is detected using biotinylated antibody and streptavidin-phycoerythrin. The fluorescent intensity is measured in a Luminex Instrument and is directly proportional to allergen concentration.
For further information, read our Primer on MARIA™ [pdf]
See: Earle et al. High throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol, 2007; 119:428-33. |
How does multiplexing work?
- Fluorescent multiplex arrays using Luminex xMAP® technology are based on polystyrene microspheres of 5.6 μm in diameter, which are labeled with internal fluorescent dyes. One-hundred bead sets can be distinguished by their internal dye ratio
- Microspheres are covalently coupled with capture monoclonal antibodies.
- During the assay, beads bearing the desired capture antibodies are combined and incubated with allergen sample. Bound allergens are detected using biotinylated mAb and a streptavidin-conjugated fluorophore.
- During measurement, microspheres are drawn into the optical path of the Luminex xMAP® instrument, where a red laser identifies beads by their internal color as bearing a specific allergen. A green laser quantifies the intensity of the external fluorescent signal, i.e. the amount of streptavidin-conjugated fluorophore, and thereby quantifies the amount of allergen bound.
- A minimum of 100 beads are counted for each allergen to obtain a highly reproducible result.
- See: Earle et al. High throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol, 2007; 119:428-33.
For further information, read our Primer on MARIA™ [pdf],
or contact: Dr. Eva King
eking@inbio.com
(1) 434 984 2304 |
Benefits of Fluorescent Multiplex Array Technology |

|
- Simultaneous measurement of multiple analytes = significantly reduced assay-time
- High-throughput: 35 samples per plate = 280 allergen data points
- Improved standardization - Assay conditions are the same for each allergen.
- All measurements are made using a single Universal Allergen Standard.
- Increased sensitivity and dynamic range of the standard curve:
- Fewer sample dilutions may be required = more samples per plate
- Results represent the median of at least 100 separate measurements per analyte = Increased reproducibility, less variation
|
Assay requirements compared
| |
ELISA |
MARIA™ |
| Samples per plate |
18 |
35 |
| Plates required |
6 plates x 8 = 48 |
3 |
| Total assay duration |
6 to 8 days |
8 hours |
Comparison is based on an allergen 8-plex on 100 samples |
MARIA™ Ordering Information
Customers can choose from two MARIA™ kits, each containing pre-mixed microspheres, as follows:
- 5-plex: Der p 1, Der f 1, Mite Group 2, Fel d 1, Can f 1 (Product Code MRA-P5)
- 8-plex: Der p 1, Der f 1, Mite Group 2, Fel d 1, Can f 1, Rat n 1, Mus m 1, Bla g 2 (Product Code MRA-P8)
Custom combinations of bead sets are available. Standard discounts (15%) are applied to orders of five or more kits - see MARIA™ pricing.
Each MARIA™ kit includes antibody coupled microspheres, biotinylated detector mAb, Universal Allergen Standard, Streptavidin-Phycoerythrin, high and low QC samples, filter plate and complete instructions for use. MARIA™ kits are licensed for use only on Luminex Corporation's laser based fluorescent analytical test instrumentation. |
| MARIA™ Services |
 Don't have access to Luminex xMAP® technology, a Luminex Instrument (or Bioplex)? Indoor Biotechnologies provides easy to use MARIA™ Service Options to analyze your environmental samples for allergens. Simply, collect a dust sample using our proprietary DUSTREAM™ collector, mail samples to Indoor Biotechnologies laboratory and our experienced analysts will provide complete 8-plex allergen array results within five days: easy, efficient and cost effective. [pdf] Don't have access to Luminex xMAP® technology, a Luminex Instrument (or Bioplex)? Indoor Biotechnologies provides easy to use MARIA™ Service Options to analyze your environmental samples for allergens. Simply, collect a dust sample using our proprietary DUSTREAM™ collector, mail samples to Indoor Biotechnologies laboratory and our experienced analysts will provide complete 8-plex allergen array results within five days: easy, efficient and cost effective. [pdf] |
| DUSTREAM™ + MARIA™ Service Options = IAQ Allergen Exposure Assessment. |
| Universal Allergen Standard for MARIA™ and ELISA |
 The standards previously used in ELISA kits were not suitable as standards in multiplex arrays. We have formulated a single Universal Allergen Standard (UAS) that contains eight purified natural allergens and is designed for use in both MARIA™ and ELISA [UAS pdf]. The standards previously used in ELISA kits were not suitable as standards in multiplex arrays. We have formulated a single Universal Allergen Standard (UAS) that contains eight purified natural allergens and is designed for use in both MARIA™ and ELISA [UAS pdf].
From January 2008, INDOOR Biotechnologies is providing the UAS in MARIA™ kits and ELISA kits (where applicable). This change to the UAS may influence allergen measurements in ongoing studies and diagnostic and allergen vaccine products.
Vial Replacement Program
To assist customers in changing to the UAS, Indoor Biotechnologies has implemented a free vial replacement program. Under this program, vials of ELISA allergen standards in ELISA kits that were purchased after July 1, 2007 will be replaced free of charge with one vial of the UAS for each ELISA kit purchased.
To take advantage of this offer, customers should provide: invoice or other documentation of ELISA kit purchase; number of ELISA kits purchased; ELISA kit product codes; and number of replacement vials being requested. US customers should contact: mail@inbio.com : other customers should contact our UK office at: info@indoorbiotech.co.uk. |
|
|
|



.jpg)